Table of Contents
What is the WFD(SCIP) Directive
The SCIP database, which stands for Substances of Concern In articles as such or in complex objects (Products), was established by the European Chemicals Agency (ECHA) under the Waste Framework Directive (WFD). The SCIP database is a pivotal tool in the EU's legislative framework, designed to manage and track hazardous substances throughout a product's lifecycle, including at the waste stage. SCIP database submissions are mandatory for any company placing products containing Substances of Very High Concern (SVHCs) on the EU market, ensuring compliance with the SCIP directive.
Understanding the SCIP Requirements for Submission
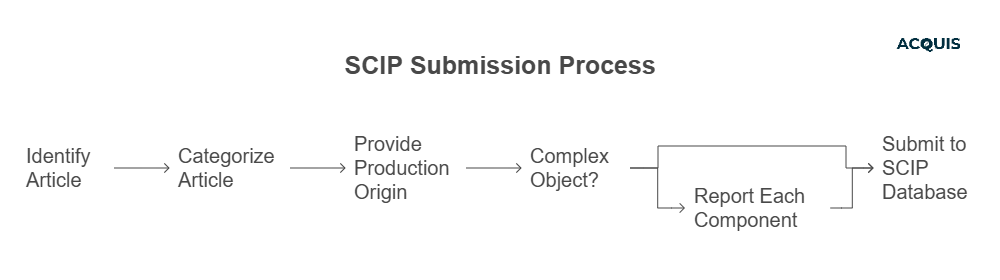
Submitting to the SCIP database involves understanding several key technical requirements:
-
Primary Article Identifier: Each article or component must have a unique identifier, such as a part number or trade name. This identifier is crucial for maintaining traceability within the SCIP database.
-
Article Category: The article must be categorized using CN or TARIC codes, which help in classifying the article correctly within the database and aligning it with EU customs and trade regulations.
-
Production Origin and Supply Chain Details: Companies must provide information about the origin of production (whether the article is produced or assembled within the EU or imported). This detail helps in understanding the article’s supply chain and its regulatory implications.
-
Hierarchical Reporting for Complex Objects: For complex objects, each component that contains an SVHC must be reported individually. The SCIP notification should clearly establish the hierarchical relationships between components to enable efficient and safe waste management practices.
How to Submit to the SCIP Database?
Submitting information to the SCIP database is a detailed process that requires precise and accurate data on the hazardous substances present in products. Here's how companies can navigate this process:
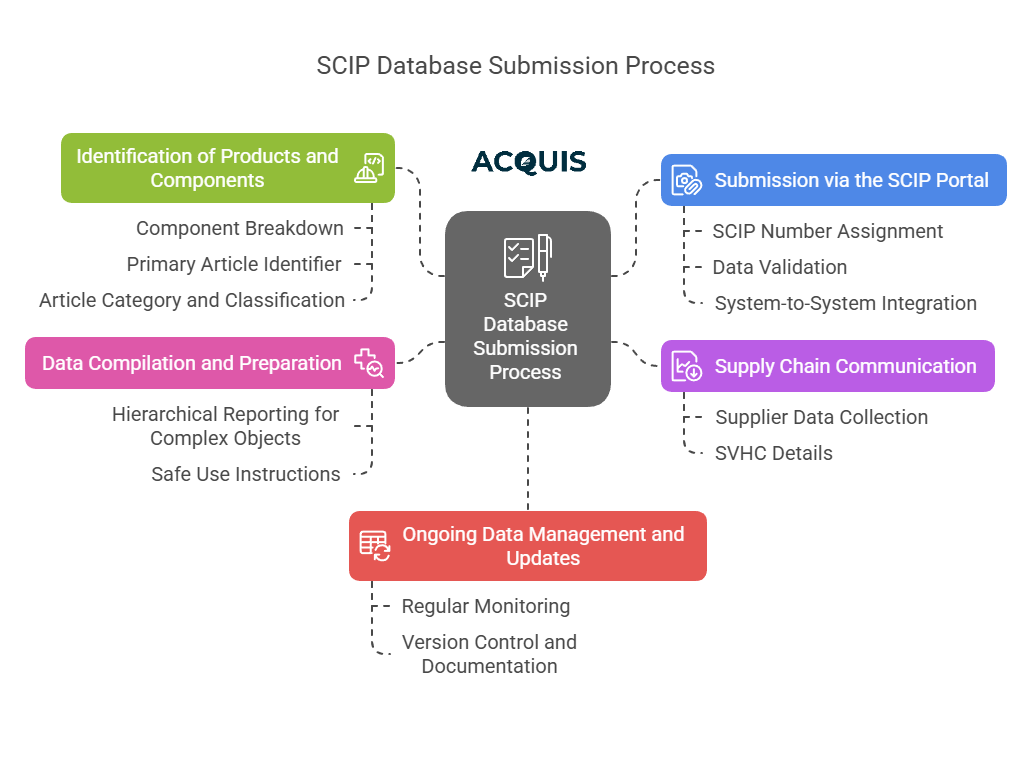
1. Identification of Products and Components
Before making a SCIP submission, companies must identify all articles and complex objects that contain SVHCs above the 0.1% threshold. This includes:
-
Component Breakdown: Each product must be broken down into its individual components and sub-components. Every component containing SVHCs must be identified and documented.
-
Primary Article Identifier: Assign a unique identifier to each article, such as an internal product code, EAN, or UPC. This ensures that each article is easily traceable within the SCIP database.
-
Article Category and Classification: Each article must be categorized using the appropriate CN/TARIC codes, which are essential for classifying the article within the SCIP database and aligning it with EU trade regulations.
2. Supply Chain Communication
Effective supply chain communication is critical for accurate SCIP reporting. Companies need to gather detailed information from their suppliers regarding the materials and substances used in their products. This includes:
-
Supplier Data Collection: Engage with suppliers to collect data on SVHCs, ensuring the accuracy of information provided for SCIP submissions. This may involve using standardized formats like IPC-1752A for data exchange.
-
SVHC Details: Collect and verify details about each SVHC present in the article. This includes the chemical name, CAS number, concentration range, and material or mixture category (e.g., polymer, metal alloy, adhesive).
3. Data Compilation and Preparation
Once all necessary information is collected, it must be compiled and prepared according to ECHA's guidelines:
-
Hierarchical Reporting for Complex Objects: For complex objects, each component containing SVHCs must be reported separately. The SCIP notification should clearly establish the hierarchical relationships between components, ensuring that the entire product structure is accurately represented.
-
Safe Use Instructions: Provide detailed safe use instructions if the SVHC poses any risk during the product's lifecycle, including disposal. This information is essential for waste operators and other stakeholders to handle the product safely.
4. Submission via the SCIP Portal
The next step involves submitting the compiled data through the SCIP portal:
-
SCIP Number Assignment: Upon submission, each article or complex object is assigned a unique SCIP number. This number is crucial for tracking and managing the article within the SCIP database.
-
Data Validation: Before submission, ensure that all data fields are accurately completed. Use the ECHA's validation tools to check for any errors or omissions in the submission, which could lead to non-compliance.
-
System-to-System Integration: For large organizations, consider implementing system-to-system integration, allowing for direct data transfer from company systems to the ECHA portal via APIs. This streamlines the submission process and reduces the risk of manual errors.
5. Ongoing Data Management and Updates
SCIP submissions require continuous updates to ensure compliance with evolving regulations:
-
Regular Monitoring: Companies must regularly monitor changes in their products and the SCIP database, updating their submissions whenever there is a change in composition, such as the addition of new SVHCs or the replacement of existing ones with safer alternatives.
-
Version Control and Documentation: Maintain thorough records of all updates to SCIP submissions. This includes keeping track of each version and documenting the changes made, which is essential for audits and compliance verification.
Download our SCIP WDF eBook here to understand the Process in detail
Acquis Automation Solution for SCIP WFD directive
The complexities of SCIP compliance can be challenging for many companies. Acquis Compliance offers expert assistance and comprehensive solutions to streamline this process and ensure full compliance with EU regulations.
Expert Consultation and Guidance
- Regulatory Expertise: Our team of compliance experts possesses in-depth knowledge of the SCIP directive, REACH regulations, and other related EU chemical compliance requirements.
- Customized Compliance Strategies: We develop tailored strategies that align with your company's specific products, supply chains, and operational processes, ensuring efficient and effective compliance.
- Training and Education: Acquis provides comprehensive training sessions for your staff, enhancing their understanding of SCIP requirements and empowering them to manage compliance processes confidently.
Comprehensive Data Management Services
- Data Collection and Analysis: We assist in gathering and analyzing all necessary data from your supply chain, identifying SVHCs, and ensuring information accuracy and completeness.
- Supply Chain Coordination: Acquis facilitates effective communication and collaboration across your supply chain, leveraging standardized data exchange formats and protocols for seamless information flow.
- Data Validation and Verification: Our experts conduct rigorous checks to validate and verify all collected data, mitigating risks of non-compliance due to inaccurate or incomplete information.
SCIP Submission Preparation and Execution
- Dossier Preparation: We handle the preparation of detailed SCIP dossiers using IUCLID software, ensuring all submissions meet ECHA's stringent formatting and content requirements.
- Efficient Submission Process: Acquis manages the entire submission process through the SCIP portal, including account setup, data uploading, and handling any technical issues that may arise.
- Error Resolution and Follow-up: In case of submission errors or inquiries from ECHA, our team promptly addresses and resolves issues, maintaining smooth compliance operations.
Ongoing Compliance Support and Maintenance
- Regulatory Monitoring: We continuously monitor updates to EU chemical regulations and the Candidate List of SVHCs, proactively informing you of relevant changes and necessary actions.
- Periodic Updates and Re-submissions: Acquis manages regular updates to your SCIP submissions, reflecting any changes in product compositions or regulatory requirements to ensure sustained compliance.
- Audit Support and Documentation: We provide comprehensive documentation and support for internal and external audits, demonstrating your company's commitment and adherence to compliance obligations.
Integrated Compliance Solutions
- Software Tools and Platforms: Acquis offers advanced compliance software solutions that integrate seamlessly with your existing systems, automating and streamlining data management and reporting processes.
- Cross-regulation Compliance: Beyond SCIP, we assist with compliance across various regulatory frameworks, including REACH, RoHS, and CLP, providing a holistic approach to chemical compliance management.
- Risk Assessment and Mitigation: Our services include thorough risk assessments of your products and processes, identifying potential compliance issues, and implementing effective mitigation strategies.
By partnering with Acquis Compliance, companies can navigate the complexities of SCIP database submissions with confidence and ease, ensuring full compliance with EU regulations while promoting transparency, sustainability, and corporate responsibility. For assistance with SCIP database submissions or questions about the process, contact our compliance experts at Acquis for guidance through every step.
