Table of Contents
Why REACH Mistakes Still Happen in 2025
Despite the maturity of the EU REACH regulation, many companies, especially those outside the EU, continue to face major compliance challenges. With regulatory updates, supply chain complexity, and evolving obligations, even well-intentioned manufacturers and OEMs can stumble. Let's break down the most common REACH compliance mistakes and learn actionable strategies to avoid them.
#1 Ignoring Article 33 Communication Obligations
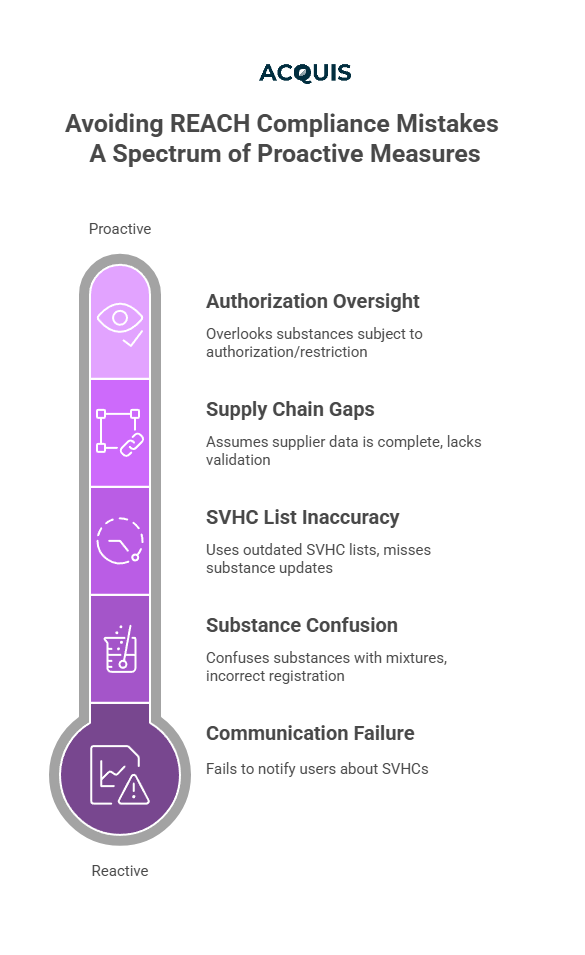
Mistake: Failing to notify downstream users and consumers about the presence of SVHCs in articles above the 0.1% w/w threshold.
Fix: Implement automated alerts when SVHCs are present in BOMs. Ensure that you send Article 33 notifications with clear usage and safety instructions.
#2 Misunderstanding "Substance" vs. "Mixture"
Mistake: Confusing individual substances with mixtures or articles, leading to incorrect registration and communication responsibilities.
Fix: Conduct REACH training for regulatory and product teams. Use material classification tools to tag each component correctly.
#3 Failing to Keep Up with SVHC Updates
Mistake: Using outdated SVHC lists and missing new substances added every January and July.
Fix: Subscribe to ECHA’s updates and integrate real-time regulatory feeds into your compliance software.
#4 Incomplete Supply Chain Declarations
Mistake: Assuming tier-1 suppliers have complete material data, or failing to validate it.
Fix: Use a digital supplier engagement tool to collect, validate, and audit material declarations (e.g., FMDs, SDS, IPC-1752A).
#5 No System for Tracking Authorisation or Restriction
Mistake: Overlooking substances that are subject to Authorisation (Annex XIV) or Restriction (Annex XVII).
Fix: Map substances in your product inventory against Annex XIV/XVII and automate alerts for changes in status or sunset dates.
The Cost of Non-Compliance: Fines, Market Access, and Reputation
From six-figure penalties to blocked shipments and public non-compliance listings, the cost of getting REACH wrong is steep. And with enforcement tightening across the EU, the margin for error is shrinking.
How Acquis Helps You Stay REACH-Ready
Acquis automates the tedious, error-prone parts of REACH compliance:
- Real-time SVHC list monitoring
- Automated Article 33 and SCIP dossier generation
- Supplier engagement workflows
- Annex XIV/XVII tracking
Let us help you simplify REACH compliance and reduce regulatory risk.
Book a Compliance Audit Review
Is your REACH program bulletproof? Book a free strategy session with our compliance experts to find out.
