Table of Contents
The United Kingdom's Registration, Evaluation, Authorization, and Restriction of Chemicals (UK REACH) framework is instrumental in ensuring the safe use and management of substances of very high concern (SVHCs). This comprehensive blog post delves into the intricacies of the UK REACH Authorisation List, with a specific focus on Annex 14. Understanding these authorization provisions is crucial for businesses and stakeholders to navigate the regulatory landscape effectively.
Purpose of UK REACH Authorisation
The core objective of the authorisation provisions under UK REACH is to facilitate the phased replacement of SVHCs with less hazardous alternatives or technologies, whenever feasible alternatives exist. This proactive approach aims to mitigate risks associated with the use of certain substances and promote the adoption of safer alternatives.
Understanding the Basics of UK REACH
The UK REACH Candidate List of SVHCs serves as the starting point, identifying substances that warrant scrutiny due to their hazardous nature. For businesses intending to persist in using or placing SVHCs on the market beyond sunset dates, compliance with the authorization process is mandatory.
HSE's Mandate Under Article 58(3)
Article 58(3) of UK REACH entrusts the Health and Safety Executive (HSE) with the responsibility of recommending priority substances for inclusion in Annex 14. This annex is crucial, as it outlines substances subject to authorization, forming a cornerstone of the regulatory framework.
Key Steps in the Authorization Process
-
Identification of SVHCs: Keep a close eye on the UK REACH Candidate List, which dynamically evolves with substances meeting specific criteria.
-
Prioritization by HSE: The HSE, as mandated by Article 58(3), prioritizes substances for Annex 14 based on various factors, including hazardous properties and usage patterns.
-
Authorization Application: Businesses must submit a comprehensive authorization application to the HSE, including detailed information on the SVHC, its uses, associated risks, and potential alternatives.
-
Risk Assessment: The HSE conducts a thorough risk assessment, weighing the benefits of SVHC use against potential risks.
-
Public Consultation: The authorization process typically includes a public consultation period, offering stakeholders an opportunity to voice concerns and provide valuable input.
-
Decision-Making: Following the assessment and consultation, the HSE decides on whether to grant authorization. Conditions or restrictions may accompany granted authorizations.
Sunset Dates and Latest Application Deadlines
As the UK REACH regulation unfolds, businesses must prioritize compliance with sunset dates and latest application deadlines, especially for Entries 44 to 54 in Annex 14. Proactive engagement with the authorization process ensures the continuity of operations and aligns with the broader goal of transitioning to safer alternatives.
Sunset Dates and Prohibited Uses
Sunset dates serve as critical milestones, marking the point at which specific uses of SVHCs become prohibited unless explicit authorization has been granted or exemptions apply. This strategic approach encourages industries to proactively transition to safer alternatives, aligning with evolving regulatory standards.
Latest Application Dates for Entries 44 to 54
Entries 44 to 54 in Annex 14 of UK REACH introduce unique considerations for businesses. According to Article 127GA (7) & (8), the latest application dates for substances within these entries are set at 30 June 2022. This signifies a pressing deadline for businesses dealing with these substances, necessitating an expedited approach to submit their authorization applications.
Key Considerations
-
Phased Transition: The phased approach mandated by sunset dates underscores the regulatory commitment to fostering a gradual shift toward safer alternatives, promoting sustainability and minimizing environmental and health risks.
-
Authorization Imperative: Businesses dealing with SVHCs specified in Entries 44 to 54 must recognize the urgency of obtaining authorization. Non-compliance with the latest application deadlines may result in prohibited uses, potentially disrupting operations.
-
Expedited Application Process: Given the time-sensitive nature of the latest application dates, businesses need to streamline their authorization application processes, involving thorough documentation, risk assessments, and proactive engagement with regulatory authorities.
-
Continuous Monitoring: Stay abreast of updates and guidance from relevant regulatory bodies. The landscape of UK REACH is dynamic, and continuous monitoring is essential to adapt to any changes in deadlines, requirements, or procedures.
Transitional Applications for Authorisation Under UK REACH
The procedure under UK REACH that gradually phases out the use of hazardous substances of very high concern is called authorization (SVHCs). It guarantees the gradual substitution of safer substitutes for these SVHCs. When the requirements are satisfied, authorization is granted, allowing the temporary use of substances on the authorization list. Transitional measures for authorisation applications submitted under EU REACH by GB-based companies are included in UK REACH. The application may be sent straight to the Secretary of State for Environment if it was in the final decision stage before the conclusion of the transition period. Transitional applications for authorisation submitted to the Secretary of State for Environment, Food and Rural Affairs.If an application is being submitted for a use that was not previously covered by an existing application, or the user was not an existing downstream user (a user before the end of the transition period) of a non GB held authorisation then the full authorisation process must be followed.
Transitional measures
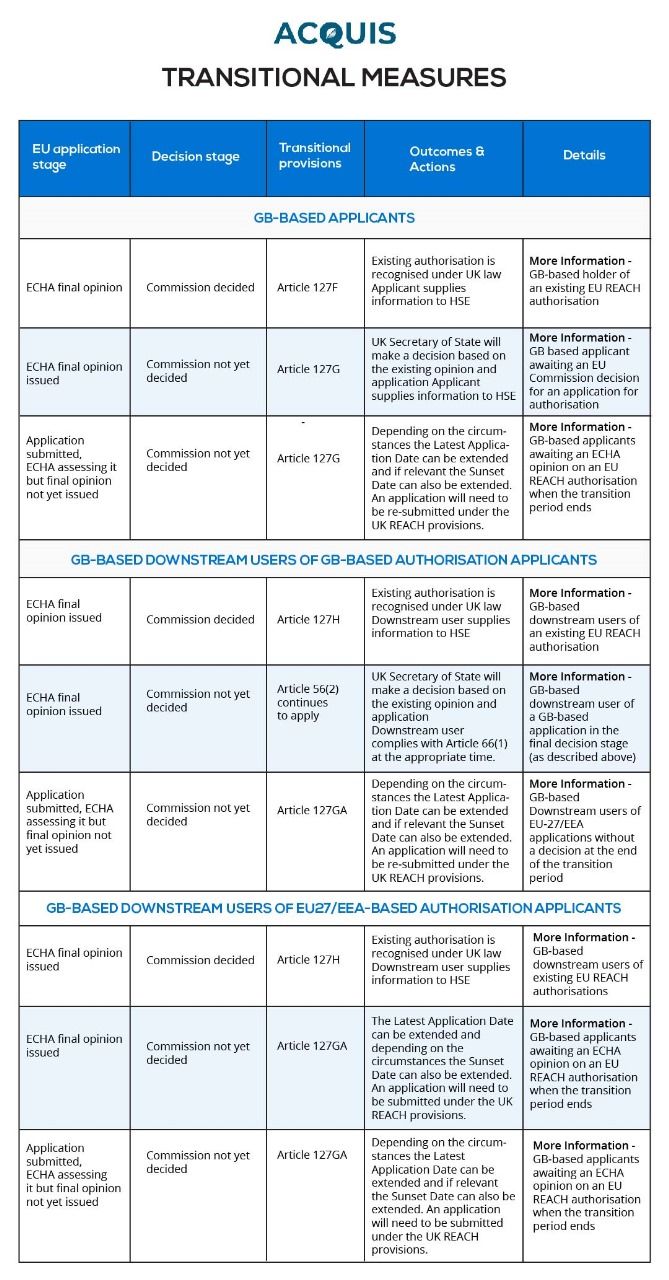
Applications for Authorisations
For businesses dealing with substances of very high concern (SVHC) under UK REACH, understanding the authorisation process is key. Fortunately, the process bears a striking resemblance to the EU counterpart, with a wealth of guidance available from the European Chemicals Agency (ECHA).
Initial Contact
Initiate the process by reaching out to the UK Health and Safety Executive (HSE) at ukreach.authorisation@hse.gov.uk. Your first point of contact sets the tone for effective collaboration. Use the subject line "notification of intention to submit an application for authorisation" to clearly convey your purpose.
The following information should be provided:
- Foreseen submission date
- The Substance(s) and use(s) for which the application will be made
- The applicant(s) and role(s) in the supply chain
- Contact details
HSE's Role in Recommending Priority Substances
A strong and adaptable chemical regulatory framework is demonstrated by the HSE's recommendation of priority substances. By identifying and prioritizing substances with higher risks, the HSE contributes to a safer and more sustainable chemical industry in the United Kingdom. According to Article 58(3) of UK REACH, the HSE is entrusted with the responsibility of recommending priority substances for inclusion in Annex 14 from the UK REACH Candidate List. This mandate emphasizes a rigorous risk assessment procedure in which the HSE assesses scientific data to identify possible risks related to substances of very high concern (SVHCs). The HSE is guided in identifying substances that require additional investigation and possibly authorization requirements by the prioritization criteria, which include aspects such as toxicity, persistence, and bioaccumulation. The HSE contributes to a chemical industry that places a high priority on safety, sustainability, and innovation by ensuring that its recommendations are in line with the larger objectives of UK REACH through open decision-making and stakeholder engagement. In the end, the HSE's proactive role in designating priority chemicals reaffirms the dedication to a more responsible and safe chemical environment in the wake of Brexit.
Notes for the Authorization List
It's crucial to note that Annex 14 of UK REACH mirrors Annex XIV of EU REACH as it stood at the end of the transition period. The same latest application dates and sunset dates apply, except for entries 44 to 54. Entries 44 to 54 have unique latest application dates of 30 June 2022, as stipulated in Article 127GA (7) & (8). Businesses must be aware of these details to ensure compliance.
Impacts of UK REACH on International Chemical Regulations :
The global context of the UK REACH authorization process (Annex 14) is crucial for businesses with international operations. Here are key points to consider:
International Regulatory Alignment
- EU REACH Comparison:
The UK REACH framework shares commonalities with the European Union's REACH. However, post-Brexit, there are divergences in regulatory processes. Businesses operating in both regions must navigate these differences.
- Harmonization Challenges: The lack of regulatory harmonization between the UK and the EU might pose challenges for businesses with cross-border operations. Divergent regulations may require tailored compliance strategies.
Impacts on Global Supply Chains
- Global Trade Dynamics: Evaluate how UK REACH impacts global supply chains. Businesses exporting to the UK or using UK-based suppliers need to align with UK REACH requirements, potentially restructuring supply chain strategies.
- Third-Country Considerations: Businesses outside the UK or EU trading blocs may face unique challenges. Understanding how UK REACH affects third-country entities ensures comprehensive compliance across the entire supply chain.
Trade Agreements and Collaboration:
- UK Trade Agreements: Explore how UK REACH fits into international trade agreements. Assess whether the UK has negotiated specific chemical regulatory provisions within trade agreements, impacting businesses involved in such agreements.
- Global Collaboration: Consider collaborative efforts between the UK and other nations on chemical regulations. Harmonizing standards with key trading partners can streamline compliance for businesses with global reach.
International Reporting and Communication:
- Cross-Border Reporting: Address the complexities of cross-border reporting. Businesses must understand reporting requirements not only under UK REACH but also consider how this aligns with reporting obligations in other jurisdictions.
- Data Sharing Mechanisms: Evaluate data-sharing mechanisms between the UK and international regulatory bodies. Efficient data exchange facilitates smoother compliance and supports global chemical safety.
Conclusion : In conclusion, navigating the UK REACH Authorization process, particularly Annex 14, is critical for businesses dealing with substances of very high concern. Understanding the phased approach, latest application deadlines, and the role of the HSE is essential for compliance. Businesses should prioritize proactive engagement, expedited application processes, and continuous monitoring to ensure a smooth transition to safer alternatives. The impact on global operations emphasizes the need for harmonization, strategic supply chain restructuring, and consideration of international trade agreements. Staying informed and adapting to the dynamic regulatory landscape will be key to achieving a more responsible and safe chemical environment in the post-Brexit era.
