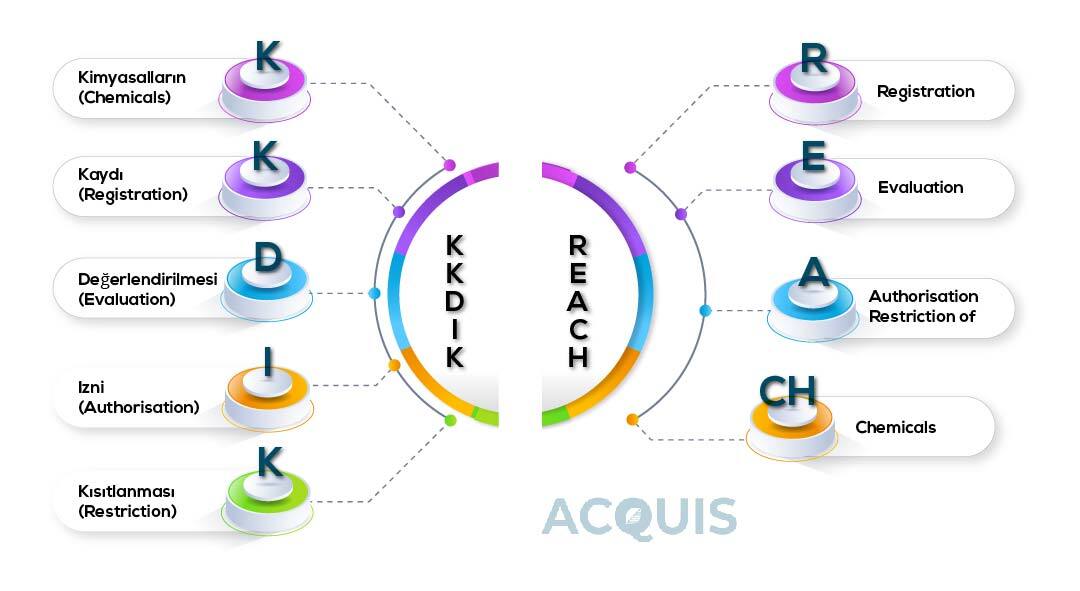
KKDIK, standing for "Kimyasalların Kaydı, Değerlendirilmesi, İzni ve Kısıtlanması" in Turkish and translated to "Registration, Evaluation, Authorization, and Restriction of Chemicals" in English, serves as Turkey's national regulation (TURKEY REACH) for the comprehensive management of chemicals. Enforced on June 23, 2017, KKDIK is consistent with the concepts of REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) and replaced three pre-existing regulations covering chemicals and mixes.
The major goal of KKDIK is to control the chemical inventory in Turkey, assuring safe production, use, and risk management of hazardous compounds. This rule is critical for preserving human health, and the environment, and avoiding the spread of hazardous substances in the Turkish market.
Similar to REACH, KKDIK aims to create a comprehensive database through the chemical registration of compounds used in Turkey. It enables the evaluation of related hazards and may grant permission to use certain hazardous compounds where the benefits outweigh the dangers. Furthermore, KKDIK has rules to ban the use of specified hazardous compounds that comply with environmental and health concerns.
KKDIK, commonly known as Turkey REACH, is vital to the proper regulation of chemical compounds in Turkey. Its complex approach includes chemical inventory monitoring, the implementation of safe practices, risk management for hazardous compounds, and the promotion of alternatives to such substances and procedures. Through its implementation, KKDIK makes an important contribution to promoting responsible chemical management and creating a safer, more sustainable environment in the Turkish market.
Why is KKDIK – Turkey REACH Important
KKDIK (Registration, Evaluation, Authorization, and Restriction of Chemicals) holds paramount importance. This Turkish REACH regulation closely resembling the EU's REACH ensures the safe production and use of chemicals within articles, which are everyday items. By requiring manufacturers to register and assess chemicals used in their products, KKDIK guarantees that articles introduced to the market are free from harmful substances, safeguarding consumer health and the environment. Additionally, KKDIK encourages the adoption of safer alternatives, fostering innovation and responsible manufacturing practices. This regulation is a crucial tool in ensuring transparency, accountability, and sustainability in the production of articles, benefiting both businesses and society as a whole.
Who Can Register Under KKDIK – Turkey REACH
The registration process under KKDIK includes providing detailed information regarding the chemical identification, properties, uses, and potential dangers of chemicals. This critical data helps authorities to assess substance safety and adopt risk management strategies. The following organisation can register with KKDIK:
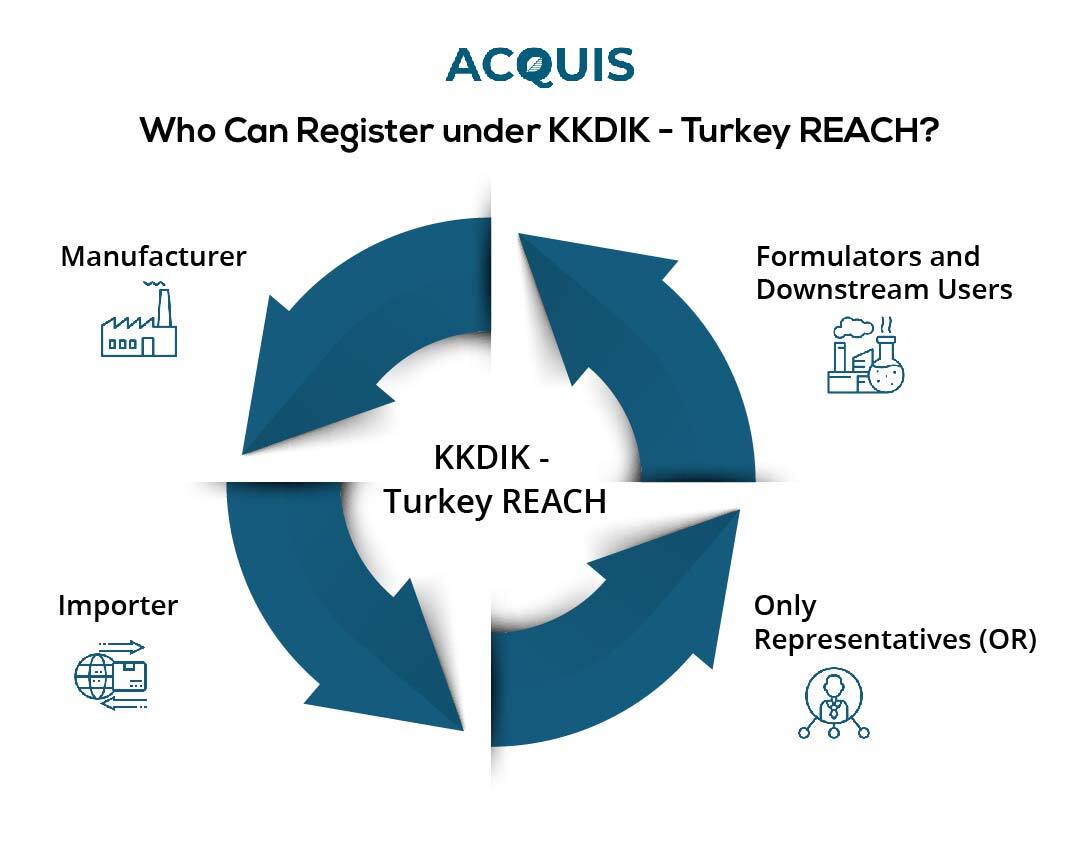
- Manufacturers: Producers of chemical substances of very high concern in Turkey are primary registrants responsible for furnishing comprehensive data about their produced substances.
- Importers: Companies importing chemical substances into Turkey can register under KKDIK if they are the first to introduce the substance to the Turkish market, ensuring compliance with safety and regulatory standards.
- Only Representatives (OR): Companies based outside Turkey can appoint an Only Representative to fulfill KKDIK registration obligations on their behalf, a common practice for non-Turkish manufacturers or importers wanting compliance without a physical presence in Turkey.
- Formulators and Downstream Users: While not always mandatory for registration, formulators (companies creating mixtures) and downstream users (employing chemicals as raw materials) play crucial roles in communicating safe handling instructions, using registered substances responsibly, and ensuring downstream compliance
Phases in KKDIK – Turkey REACH:
KKDIK phases are designed to progressively assess and manage the risks associated with chemical substances. Here are the three main phases of KKDIK’s chemical registration program-
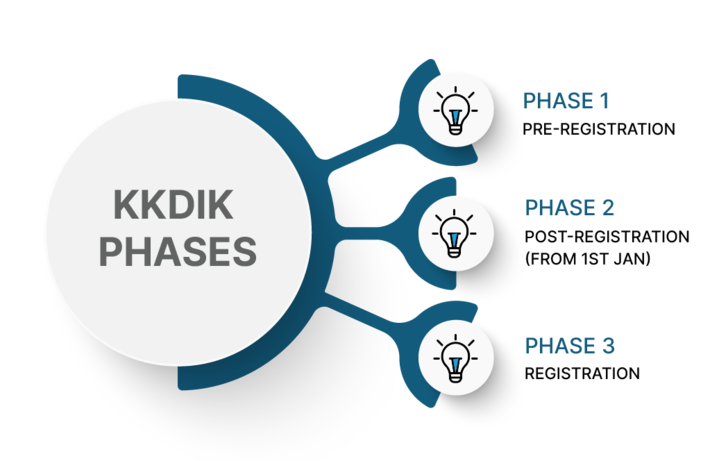
- Pre-registration Phase: This phase is similar to pre-registration in REACH-IT. According to Article 25(1) of KKDIK: “All potential registrants, downstream users and third parties of substance who have submitted pre-substance information exchange forum (pre-SIEF) to the Ministry, or registrants who have submitted a registration for that substance before 31/12/2023 shall be participants in a substance information exchange forum (SIEF).”
All pre-registrations should be submitted before 31 December 2020 along with the information on chemical substances identity according to Annex VI and registrants role in the supply chain through the online Chemicals Registration System (KKS – Kimyasal Kayıt Sistemi) in the website of the Ministry of Environment and Urbanization (MoEU).
-
Phase of Registration: Preregistered substances may be manufactured, imported, or placed on the market within the country in this phase. Non-pre-registered substances may only be manufactured, or imported or placed on the market within the country after the registration process is completed. The substances for which preregistration procedures have been completed must be submitted along with a compiled dossier to the Ministry by the certified Chemical Assessment Expert described in KKDIK Annex XVIII for each substance between 1 January 2021 and 31 December 2023.
-
Post-registration(from 1st jan) : After the completion of the registration phase on 1 January 2024, substances that have not been previously registered may only be manufactured or imported, or placed on the market within the country after registration has been completed. In other words, as of 2024, companies
What is the Regulatory Scope of KKDIK – Turkey REACH
Chemical substances, whether alone or in combinations, or intended for release in articles, must be registered with the MoEU in quantities equal to or greater than 1 tonne per year, similar to the EU REACH law. Companies must also meet the requirements of the Evaluation, Authorization, and Restriction (EAR) and the safety data sheet (SDS).
Replaced Laws by KKDIK :
- Regulation on the Inventory and Control of Chemicals, No. 27092 (replaced on 23 June 2017),
- Regulation on the Restrictions Relating to the Production, Supply to the Market and Use of Certain Hazardous Materials, Products and Goods, No. 27092 (replaced on 23 December 2017),
- Regulation on the Preparation and Distribution of Safety Datasheets for Hazardous Materials and Products, No. 29204 (replaced on 31 December 2023).
Exemptions from KKDIK - Tukey REACH:
- Radioactive substances: Exempt from KKDIK Regulation, likely regulated under specific laws for radioactive materials.
- Substances subject to customs supervision: Exempt, with regulations governing import, export, and handling separately.
- Substances for use in national defense: Exempt due to critical national defense roles, potentially governed by separate regulations.
- Wastes: Exempt, with waste management likely covered by distinct environmental laws.
- Non-isolated intermediates: Exempt to avoid redundant registration during intermediate stages of chemical processes.
- Carriage of hazardous substances: Exempt for transportation, regulated separately for rail, road, inland waterway, sea, or air.
Deemed as KKDIK - Tukey REACH Registered Substance :
- Active substances for use in Biocidal Products: Automatically considered registered under specific regulations for biocidal products.
- Active substances for use in Plant Protection Products: Deemed registered for use in plant protection products under relevant regulations.
Exempted from KKDIK Tukey REACH Regulation Registration:
- Foods or feeding stuff: Exempt from KKDIK Regulation, possibly governed by food safety and labeling regulations.
- Medicinal products: Exempt, likely regulated under specific pharmaceutical laws.
- Substances included in Annex IV: Exempted substances listed in Annex IV of KKDIK Regulation.
- Substances included in Annex V: Exempted substances listed in Annex V of KKDIK Regulation.
- Registered substances from the recovery process: Exempt if derived from a recovery process, regulated separately.
- Registered substances being re-imported: Exempt if substances are re-imported, with specific conditions.
- Polymers: Monomers and reactants need registration, but polymers as a whole are exempt.
- PPORD substances (PPORD notification): Exempted under the Pre-registration of New Substances on their Own or in Mixtures (PPORD) notification process. Top of Form
KKDIK Registration and Obligations in Turkey
KKDIK (Turkish REACH) requires manufacturers and importers of chemical substances to register them if they're produced or imported in quantities of one ton or more per year. KKDIK registration includes two stages, namely Pre-SIEF (Pre-registration) and Registration. This KKDIK Registration program involves providing detailed information about the chemical substances, their uses, properties, and potential risks. This process ensures transparency, informed decision-making, and safe chemical management. Pre-registration is optional and offers extended registration deadlines. Joint registrations can be done for the same substances, sharing data and resources. Non- Turkish entities can use Only Representatives. Registration is vital for promoting responsible chemical usage and protecting human health and the environment.
The pre-registration started on December 23rd, 2017 and all pre-registrations should be submitted before December 31st, 2020. The Registration Period will start on December 31st, 2020, and if the Pre-Registration has been completed by December 31st, 2020, chemical substances can be manufactured in Turkey or imported into the country until December 31st, 2023. After December 31st, 2023, substances that have not been registered under KKDIK cannot be manufactured in Turkey or imported into the country with a volume equal to or above 1 tonnage per year.
Registration Obligation:
Since the responsibility for fulfilling the processes mentioned in KKDIK belongs to the manufacturer, importer, downstream user or their only representative, these bodies are obliged to ensure that the chemical substances which are manufactured and used are not harming human health and the environment. With this aim, KKDIK introduces a registration obligation. There are some exemptions to the registration obligation :
- Substances Outside KKDIK Scope: Substances not covered by KKDIK, such as radioactive materials, substances under customs supervision, those for national defense, wastes, non-isolated intermediates, and hazardous substances transported by rail, road, inland waterway, sea, or air, are exempt from pre-registration or registration obligations.
- Exemptions Within KKDIK Scope: Substances within KKDIK's scope may be exempt from pre-registration or registration requirements. This includes foods, feeding stuff, medicinal products, substances listed in Annex IV and Annex V, registered substances obtained from the recovery process, substances being re-imported, polymers (with a registration requirement for monomers and other reactants), and substances subject to Prior Informed Consent (PIC) regulations.
- Substances Considered Registered: Active ingredients within specific product categories, such as active substances used in biocidal products and plant protection products, are deemed registered under KKDIK. Consequently, there is no need for additional registration for these substances.
Scope of SDS in KKDIK Turkey REACH:
The Safety Data Sheet (SDS) plays a crucial role in managing chemical safety. It mandates that manufacturers and importers of chemical substances provide a comprehensive SDS for each product they produce or import. The current SDS regulation (No.29204) is set to be replaced by KKDIK Annex II on December 31st, 2023. During the transitional period from December 23rd, 2017, to December 31st, 2023, both regulations will be in effect.
Key SDS Requirements in Turkey include:
- Standardized 16-Section SDSs: Manufacturers and importers are obligated to provide Safety Data Sheets conforming to the standardized 16-section format.
- Language Mandate: SDSs must be prepared in Turkish, ensuring accessibility and comprehension for relevant stakeholders.
- Certified SDS Experts: The preparation of SDSs necessitates the involvement of certified SDS experts based in Turkey. This requirement underscores the importance of qualified professionals in ensuring the accuracy and reliability of safety information.
- Submission to MoEU: A crucial aspect of compliance involves submitting a copy of the SDSs to the Ministry of Environment and Urbanization (MoEU). Notably, an expert holding a current certificate is precluded from preparing new SDSs until updated guidance is received.
Crucial key points about KKDIK
- Certified Experts: Only certified Chemical Assessment Experts mentioned in KKDIK Annex XVIII of REACH are authorized to compile the dossier, CSR, and SDS/eSDS.
- Phase-In and Non-Phase-In: No differentiation between phase-in and non-phase-in substances; both existing and new substances can be pre-registered and registered.
- Registration Deadline: The registration deadline isn't linked to tonnage bands; it applies uniformly to substances regardless of production volume.
- Data Requirements: The data needed for registration closely match those outlined in EU REACH's Annex VII, VIII, IX, and X.
- EU REACH Data Acceptance: EU REACH data can be utilized for KKDIK registration, but a new usage right might be necessary to comply with Turkey's specific conditions.
Differences between Turkey REACH and EU REACH
One of the primary differences between REACH and KKDIK is that the latter calls for the approval of registrations and notifications only by trained and qualified experts. Other than that, there are not any significant distinctions between EU REACH and Turkey's KKDIK regulation. Companies must primarily complete the following three tasks in order to comply with KKDIK regulations.
- Pre-register/register applicable substances >=1t/y before the given deadline. If your company is based outside of Turkey, you may need to appoint a local only representative.
- Ensure that your safety data sheets are compliant.
- Monitor the list of substances subject to authorization on annex 14 and the list of restricted substances on annex 17.
Extension of KKDIK-TURKISH REACH Registration Deadline
The Revision of KKDIK Regulation Regarding the Extension of Registration Deadlines has been officially published in the Official Gazette on 23 December 2023, under No: 32408.
- I Registration Deadline Extension Criteria
Registrations must be completed by 31/12/2026 for substances meeting the following conditions:
-
Substances manufactured or imported in quantities of 1000 tons or more per year, whether on their own, in mixtures, or in articles.
-
Substances manufactured or imported in quantities of 100 tons or more per year, classified as Aquatic Acute 1 and/or Aquatic Chronic 1 (H400, H410), whether on their own, in mixtures, or in articles.
-
Substances manufactured or imported in quantities of 1 ton or more per year, classified as carcinogenic, mutagenic, and toxic to the reproductive system, Category 1A and 1B, whether on their own, in mixtures, or in articles.
-
II. Deadline Extension until December 31, 2028 For substances manufactured or imported in quantities of 100 tons or more annually, either individually, in mixtures, or as components of articles, the deadline is extended until December 31, 2028.
-
III. Extended Deadline: 31/12/2030
Substances manufactured or imported in quantities of 1 ton or more per year, either individually, in mixtures, or as components of articles, have an extended deadline until December 31, 2030.
Note: Detailed provisions on pre-registration will be released by the Competent Authority, with the lifespan of SIEFs extended until 31/12/2032 . No Extension for SDS Provisions
It is crucial to highlight that there is no extension concerning the Safety Data Sheet (SDS) provisions of KKDIK. The former SDS Regulation 29204 will be repealed as of 31/12/2023. Consequently, starting from 1/1/2024, SDSs must be compiled in accordance with the provisions outlined in KKDIK Annex II.
Conclusion :
In conclusion, the recent Revision of KKDIK Regulation represents a significant step forward in aligning Turkey's chemical management framework with international standards, particularly mirroring the principles of the European Union's REACH regulation. The extended registration deadlines provide a pragmatic approach, allowing industries more time to comply with Turkey’s ROHS Directive(KKDIK) while maintaining a focus on safety and environmental protection. KKDIK's emphasis on transparency, accountability, and sustainability in chemical production underscores its pivotal role in safeguarding both public health and the environment. As Turkey continues to refine its chemical regulatory landscape, collaboration between stakeholders and adherence to the revised KKDIK guidelines will be crucial for ensuring a safe and sustainable future in the handling of chemical substances within the country.
Speak to Our Compliance Experts
Share
ENVIRONMENTAL COMPLIANCE
- RoHS
- SCIP (WFD)
- REACH
- California Proposition 65
- Material Disclosure (FMD)
- PFAS
- TSCA PBT
- EU POPs
- EU MDR & IVDR
- ELV (GADSL)
- Others
- Extended Producer Responsibility (EPR)
INTEGRATION SUPPORT
WE ARE GLOBAL
USA
6705 Ridgedale CT, Glen Allen, VA 23059
+1.757.801.2760
info@aquiscompliance.com
India
#9/2, Hennur Bagalur Main Road, Bengaluru - 560077
+91 789 238 1827
info@aquiscompliance.com
