EU Revises CLP Regulation: Regulation (EU) 2023/707 Amending Regulation (EC) No 1272/2008
On 31 March 2023, the European Commission published the delegated act (Regulation (EU) 2023/707) amending Regulation (EC) No 1272/2008 as regards hazard classes and criteria for the classification, labelling, and packaging of substances and mixtures.
Introduction to CLP Regulation
The Classification, Labelling and Packaging (CLP) Regulation is a European Union regulation that sets out criteria for identifying and classifying the hazards of chemical substances and mixtures. The CLP Regulation applies to manufacturers, importers, and downstream users of chemicals, and requires them to classify and label their products appropriately. When a substance is classified as hazardous under the CLP Regulation, it is assigned a hazard class and category. These hazard classes include physical hazards such as flammability and explosiveness, health hazards such as toxicity and carcinogenicity, and environmental hazards such as aquatic toxicity and ozone depletion.
If a proposal is made to harmonize the classification and labelling of a substance across the EU, the European Chemicals Agency's (ECHA) Risk Assessment Committee (RAC) will assess the proposal and provide an opinion. This process supports the principle of "one substance, one assessment," which aims to ensure that the hazards of a substance are assessed in a consistent and transparent manner, regardless of where it is manufactured or used in the EU. The RAC is composed of independent experts from across the EU who have expertise in the areas of toxicology, chemistry, and risk assessment. The RAC's opinion is based on a scientific assessment of the available data on the substance's hazards. The opinion is then used by ECHA to make a decision on whether or not to harmonize the classification and labelling of the substance.
The principle of "one substance, one assessment" is important because
- It helps to ensure that workers, consumers, and the environment are protected from the hazards of chemicals. By ensuring that the hazards of a substance are assessed in a consistent and transparent manner, the principle helps to prevent the unnecessary duplication of testing and assessment, and it also helps to ensure that the most up-to-date information on the hazards of a substance is available to those who need it.
- For the smooth functioning of the EU's internal market. By ensuring that the classification and labelling of chemicals is harmonized across the EU, the principle helps to prevent technical barriers to trade and it also helps to ensure that consumers have access to safe and reliable products.
The principle of "one substance, one assessment" is a key part of the EU's chemicals policy. It is a principle that is based on sound science and it is a principle that is essential for protecting workers, consumers, and the environment from the hazards of chemicals.
EU has recently introduced an amendment to the Classification, Labelling, and Packaging (CLP) Regulation, bringing about significant changes to enhance chemical safety. By understanding and complying with these revised requirements, businesses can ensure the safety of workers, consumers, and the environment, while also maintaining regulatory compliance.
New Requirements for Classification, Assessment, and Labeling
The latest amendment to the CLP Regulation introduces new requirements outlined in Annex I, focusing on the classification, assessment, and labeling of hazardous substances and mixtures. These requirements specifically target substances and mixtures falling into the following categories:
-
Endocrine Disruptors (ED) Endocrine disruptors are substances that can interfere with the normal functioning of the endocrine system, causing adverse effects on human health and the environment. Annex I now includes explicit provisions for the classification, assessment, and labeling of endocrine disruptors. By incorporating this crucial aspect, the EU aims to ensure that these substances are appropriately identified, and their potential risks are communicated effectively.
-
Persistent, Bioaccumulative, and Toxic (PBT) or Very Persistent or Very Bioaccumulative (vPvB) Substances PBT and vPvB substances are characterized by their ability to persist in the environment, accumulate in living organisms, and exhibit toxicity. The amendment introduces new requirements for the classification, assessment, and labeling of these substances. By doing so, the EU aims to address the unique hazards associated with PBT and vPvB substances, enabling better risk management and informed decision-making.
-
Persistent, Mobile, and Toxic (PMT) and Very Persistent, Very Mobile (vPvM) Substances PMT and vPvM substances pose specific challenges due to their potential to contaminate water resources and spread extensively. The revised CLP Regulation now includes provisions for the classification, assessment, and labeling of these substances. This addition empowers businesses to accurately identify and communicate the risks associated with PMT and vPvM substances, facilitating the adoption of appropriate safety measures.
Revision of Annex II: Special Rules for Labeling and Packaging
Annex II of the CLP Regulation, which focuses on the special rules for labeling and packaging, has also been revised. One significant change relates to the concentration of substances classified as Endocrine Disruptors (ED) for human health category 2 or environmental category 2. The amendment provides specific guidelines on labeling and packaging requirements for substances falling into these categories, ensuring clear and consistent communication of their hazardous nature.
Amendments to Hazard Statements and Introduction of New Codes
The latest amendment to the CLP Regulation includes revisions to hazard statements listed in Annex III. These hazard statements play a crucial role in effectively communicating the specific dangers posed by hazardous substances. By updating and aligning these statements with the latest scientific understanding, the EU ensures that individuals have access to accurate and up-to-date information about the hazards associated with chemicals.
Additionally, new hazard codes and category codes have been inserted into Annex VI. These codes specifically cover Endocrine Disruptors (ED), Persistent, Bioaccumulative, and Toxic (PBT), Very Persistent or Very Bioaccumulative (vPvB), Persistent, Mobile, and Toxic (PMT), and Very Persistent, Very Mobile (vPvM) substances. The introduction of these codes provides a standardized classification and labeling system for these hazardous substances, streamlining hazard communication across the EU.
Below are the new EU CLP hazard statements:
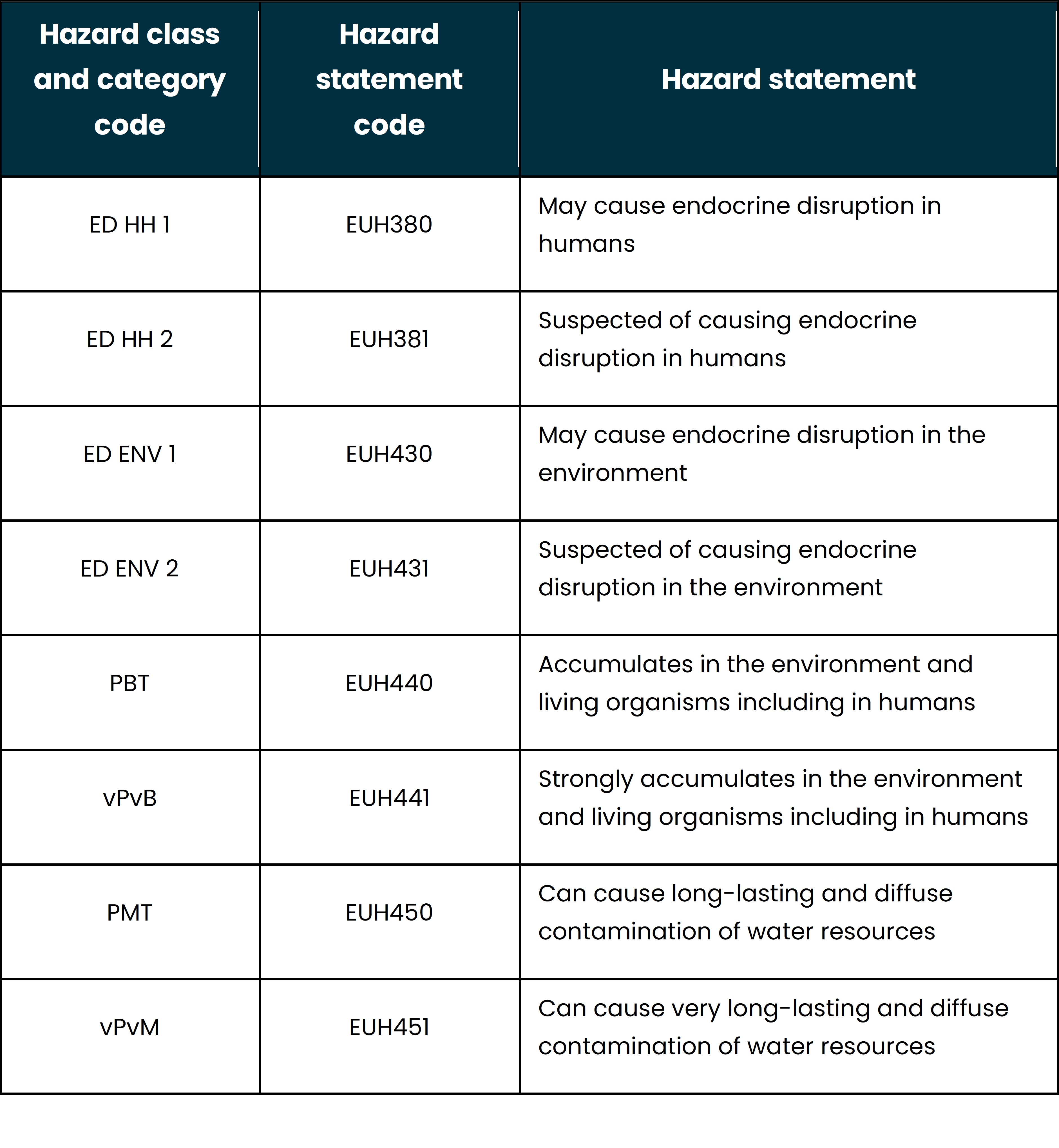
The new rules came into effect on April 20, 2023. Members can now submit proposals for harmonized classification and labelling (CLH) with the new danger classes, and manufacturers, importers, downstream users, and distributors can self-classify their substances and mixtures accordingly. From the date of the Delegated Regulation's entrance into force, makers, importers, downstream users, and distributors are not obligated to categorize their substances or mixes according to the new danger classes. During these times, the new danger classes can be applied voluntarily.
All makers, importers, downstream users, and distributors must utilize the new hazard classes after the transitory periods expire. For new substances on the market, companies need to comply with the new rules from 1 May 2025, whereas substances that have already been on the EU market, companies have until 1 November 2026 to comply.
Conclusion
The recent amendment to the CLP Regulation signifies the EU's ongoing commitment to improving chemical safety within its borders. By enhancing the classification, assessment, and labeling of hazardous substances and mixtures, the EU aims to create a safer and more transparent environment for chemical handling. Businesses play a pivotal role in ensuring compliance with these new requirements, contributing to the overall protection of workers, consumers, and the environment.
By embracing these changes, the EU continues to lead the way in prioritizing the safety and well-being of its citizens, while also establishing a strong framework for responsible chemical management. The revised CLP Regulation sets a higher standard for chemical safety, fostering trust, transparency, and accountability in the EU's chemical industry.
Speak to Our Compliance Experts
Share
ENVIRONMENTAL COMPLIANCE
- RoHS
- SCIP (WFD)
- REACH
- California Proposition 65
- Material Disclosure (FMD)
- PFAS
- TSCA PBT
- EU POPs
- EU MDR & IVDR
- ELV (GADSL)
- Others
- Extended Producer Responsibility (EPR)
INTEGRATION SUPPORT
WE ARE GLOBAL
USA
6705 Ridgedale CT, Glen Allen, VA 23059
+1.757.801.2760
info@aquiscompliance.com
India
#9/2, Hennur Bagalur Main Road, Bengaluru - 560077
+91 789 238 1827
info@aquiscompliance.com



